The following information is provided to guide faculty, staff, and students through the Cal State LA IRB process.
IRB Online Training Certificate
Cal State LA Institutional Review Board requires all researchers who will have contact with participants, data or biospecimens complete an education and training program on the protection of human research subjects. This requirement is achieved by completing the online training offered by Collaborative Institutional Training Initiative (CITI). Each researcher must provide their CITI certificate (valid for three years) upon submission of their IRB application as proof of training is a condition for IRB approval.
More information and instructions are provided on the CITI Online Training Program page.
IRBNet
All investigators who will be part of the research must have their own IRBNet account. Please visit IRBNet to register and create your IRBNet User Profile.
To streamline the submission process, the following resources provide step-by-step instructions to navigate IRBNet including specific Cal State LA IRB requirements.
To determine if a proposed research project requires IRB approval, researchers may review and complete the "Is My Project Human Subjects Research," screening form.
Students planning to conduct research with human subjects for their thesis or culminating project should consult with their faculty mentor to determine when to submit the IRB application.
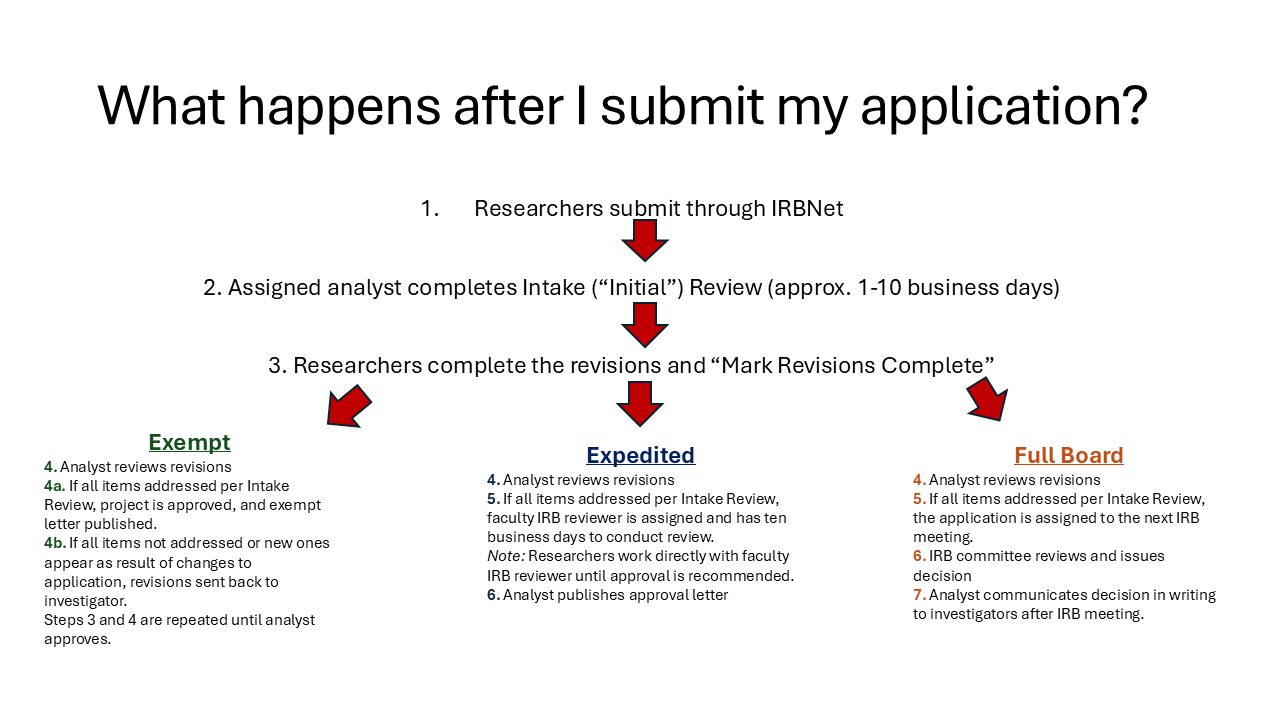
An initial intake review of all applications will be conducted to ensure that the appropriate application and related study materials have been submitted. Each type of IRB application has a slightly different review procedure. If the proposed research will require full committee review, then please refer to the IRB Meeting Schedule page for additional guidance for submission. Exempt and expedited applications are reviewed year-round.
Cal State LA IRB staff complete several types of administrative reviews outside of IRBNet. Please find more information about each one below.
IRB review and approval is not required for research projects where only unrestricted, publicly available data are analyzed. Investigators may receive an official determination from IRB staff regarding the necessity of IRB review by submitting the "Is My Project Human Subjects Research," screening form.
Collaborative research projects may be eligible for an Institutional Authorization Agreement (reliance agreement) which is an agreement signed by two or more institutions engaged in the same regulated human subjects’ research project (expedited or full board). Please contact IRB staff at irb@calstatela.edu to determine if a reliance agreement is required between Cal State LA and the other institution(s).
Oral History does not require IRB approval under the 2018 Requirements (2018 Revised Common Rule) per 45 CFR 46.
Non-affiliated investigators will need to request departmental permission to recruit Cal State LA students, faculty, or staff as research participants. The Cal State LA IRB cannot grant permission on behalf of other campus entities. If permitted, please send the department a copy of your IRB approval or exemption letter.
If you are a member of the Cal State LA community and you have questions about a request from an outside researcher, the IRB staff are available for consultation.
CITI Online Training
Cal State LA Institutional Review Board requires all researchers who will have contact with participants, data or biospecimens must complete an education and training program on the protection of human research subjects. This requirement is achieved by completing the online training offered by Collaborative Institutional Training Initiative (CITI). Each researcher must provide their CITI certificate (valid for three years) upon submission of their IRB application as proof of training is a condition for IRB approval.
CITI
New CITI Users:
To create an account, click "Register" and follow the instructions provided on the screen. Make sure to affiliate your account with "California State University Los Angeles" (Step 1). Be prepared to provide your university e-mail, your Campus Identification Number (CIN), your Role in Human Subjects Research, and a phone number (Step 6).
Returning CITI Users:
If you completed CITI training for another institution, you need to add an affiliation to Cal State LA.
- Log into CITI Program using your username and password that you created for your previous institution.
- At the bottom of the "Main Menu" page, click "Affiliate with Another Institution".
- Search for "California State University Los Angeles" and follow remaining instructions.
- Please keep in mind that you may need to complete additional modules to receive the CITI certificate under Cal State LA's requirements.
The CITI course that investigators will need to complete depends on the type of proposed research. The Cal State LA IRB will accept the “Social and Behavioral Research” or “Biomedical Research” certificates.
Once finished, please complete the following steps: